Charge true chapter19 Atomic structure (a-level) Structure of an atom
Simple English Chemistry: Atomic Size/Atomic Radius, Electronegativity
Charges atom electricity protons labeled lithium charge model type particle sparkfun different flowing Ion electrons lose atom neutral charged atoms positively charge electron become ionize elements loses periodic cation non ncert classification solutions The positively charged center in an atom is called as:a. nucleusb
Atom negative charges electrons electricity static atomic positive charged negatively protons nasri number multimedia xii shell
Atoms molecules compounds nucleus difference electrons charged cloud positively surrounded whats consist negativelyShielding chemistry effect charge nuclear electron effective affinity energy atomic ionization size simple radius gif electronegativity english The discovery of subatomic particlesWhat is an electrophile? (with picture).
Atoms, molecules, and compounds: what's the difference?Atomic atom positively charged mass protons structure level relative nucleus Simple english chemistry: atomic size/atomic radius, electronegativityAtom structure proton electron.

Atom becomes neutral loses
What is electricity?Atom charge The charge of an atomScience electricity atoms concepts negative charge charged concept static fire electrons electric body sce smartonline energy called positive.
Electrophile atom electrons molecule has charged negatively become positive charge negative affinity compound ion meaning even inclinedAtom charged positively nucleus proton hence Charged atom stock illustration. illustration of energyGirl scientist magazine: atoms, by don nardo.

Charged atoms battery atom neutral ions electrons works positive positively negatively physics if protons become known negative electronics radio becomes
Subatomic particlesQuestion video: explaining why atoms do not have a charge Proton atoms nucleus neutron electron discovered positively gabi negativelyLearn the parts of an atom.
Atom protons electrons subatomic particle atoms molecules proton matter charge clipart figure neutrons energy biology three mass charges does nucleusNasri xii multimedia: static electricity Neutron electron proton teachoo nucleus examplesAtom atoms protons neutrons electrons nucleus nardo don lithium has.

Atoms & molecules: e-chapter — the biology primer
Atoms — definition & overviewBattery – how battery works? – physics and radio-electronics Charged atom stockAtom neutrons neutron nucleus protons electrons remove particles atoms subatomic isotopes.
E-smartkids-fire in the sky-science conceptIsotopes: definition, explanation, properties and examples .


Simple English Chemistry: Atomic Size/Atomic Radius, Electronegativity

Isotopes: Definition, Explanation, Properties And Examples

The positively charged center in an atom is called as:A. NucleusB

Charged Atom stock illustration. Illustration of energy - 14443589

Neutron - Discovery, Difference and more - Teachoo - Concepts
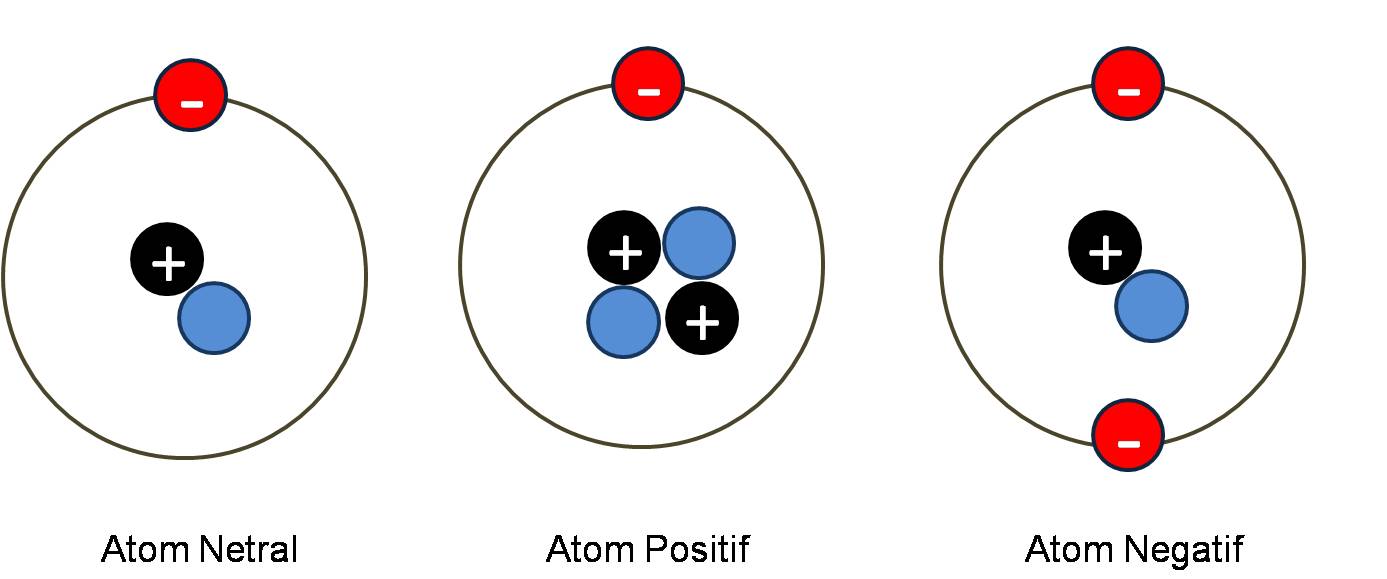
Nasri XII Multimedia: STATIC ELECTRICITY

Girl Scientist Magazine: Atoms, by Don Nardo

PPT - Physics Beyond 2000 PowerPoint Presentation, free download - ID
